 Loading... Please wait...
Loading... Please wait...Our Newsletter
- Home
- Tests
Tests
Avoiding aluminum ingestion altogether is literally impossible. That’s because aluminum is one of the most abundant elements in soil.
Here are some examples of the average amounts of aluminum found in common foods:
- Wheat and corn: 140 PPM (parts per million)
- Potatoes: 100 PPM
- Lettuce: 90 PPM
- Beans: 165 PPM
- Tomatoes: 90 PPM
- Pineapple: 100 PPM
- Bananas: 97 PPM
- Coffee: 97 PPM
You get the idea: There is no food containing zero aluminum.
Liquid Zeolite Contains less aluminum than ALL of these foods because the serving size of Liquid Zeolite is much smaller than the serving size of any of these foods. Plus, Liquid Zeolite doesn't release or leach any aluminum into the body, it carries out its' aluminum AND attracts and removes other aluminum, heavy metals & toxics found in the body.
====
3rd Party Lab Tests
Here is the most recent test results performed by an independant 3rd party lab for our Liquid Zeolite™.
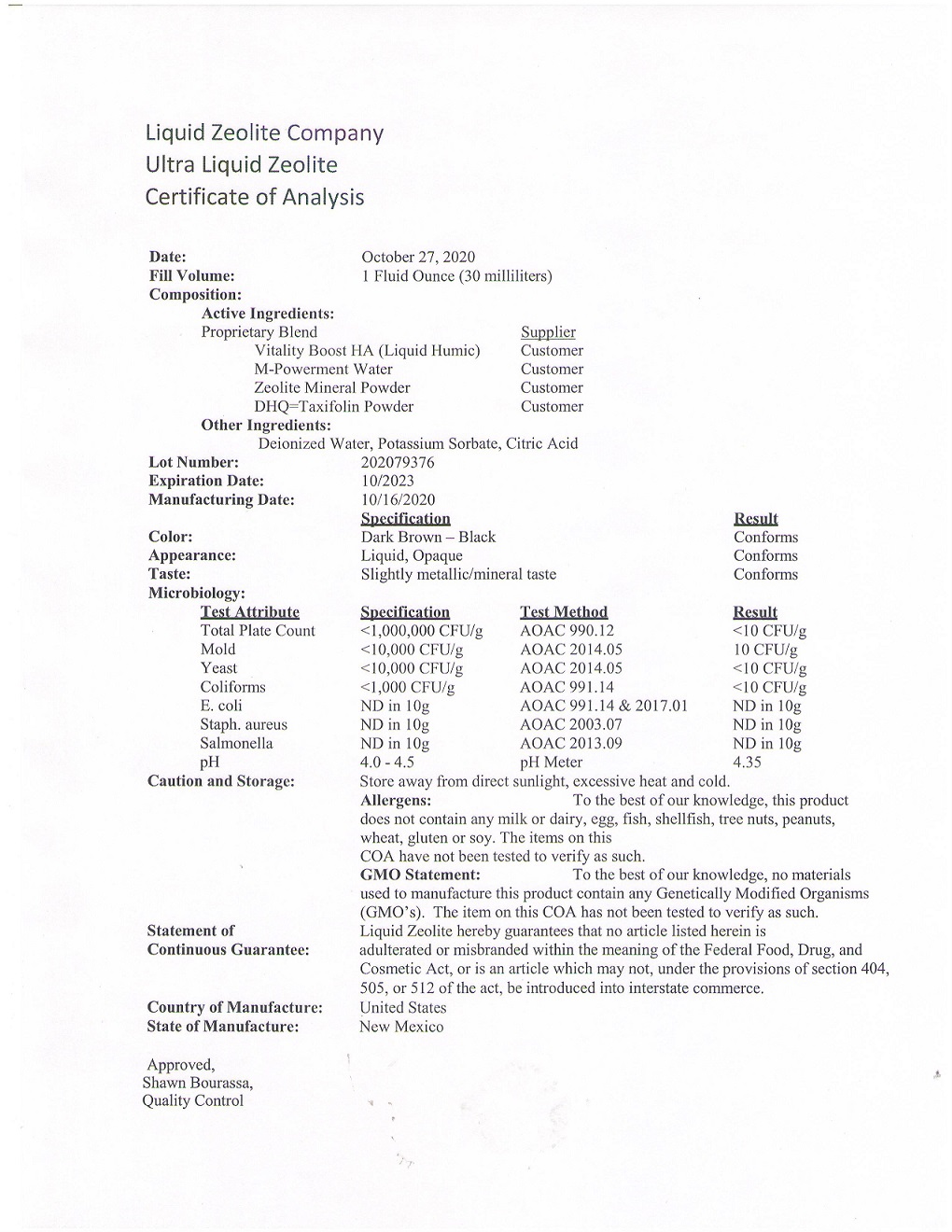
You should know that our Liquid Zeolite™ is made in a fully GMP compliant lab within the USA. Good Manufacturing Practices (GMP) Certification Program verifies to consumers that dietary supplements are manufactured according to high standards. The third-party certification program includes inspections of dietary supplement manufacturing facilities to determine whether specified performance standards on a number of measures—including quality control, cleanliness, receiving and testing of raw materials—are being met.
A multi-level marketing company that sells an over-priced liquid zeolite type products enjoys making up stories about liquid zeolite in humic acid & fulvic acid by saying it "may" contain bacteria, mold, fungus, etc. Here are five reasons why this is a ridiculous and totally untrue statement:
1. The Humic Acid/Fulvic Acid blend we use in our Liquid Zeolite™ is a high quality extraction that has been specifically manufactured, sterilized, standardized and prepared 100% safe for human consumption.
2. We heat-pasteurize our Liquid Zeolite™.
3. We add a 100% safe, natural preservative to our product.
4. We have it thoroughly tested by independent 3rd party analysis. Microbial tests are always done before any product is shipped as part of our stringent quality control measures.
5. Our Liquid Zeolite™ product is manufactured in a GMP certified and FDA licensed manufacturing facility.